Evaluation of physico-chemical characteristics of groundwater of Sindhuvalli Village, Mysore District
EVALUATION
OF PHYSICO-CHEMICAL CHARACTERISTICS OF GROUNDWATER OF SINDHUVALLI VILLAGE - MYSORE
DISTRICT
A
dissertation report submitted to Dept. Environmental science, Yuvaraja’s
college (autonomous)
MAEED
M. ZAHIR - EMMANUEL .C - FIDAAD JALEEL
Class 2012 - VI Sem - Bachelor of
Science (CEnEr)
DEPARTMENT
OF ENVIRONMENTAL SCIENCE
YUVARAJA’S
COLLEGE
UNIVERSITY
OF MYSORE
April
2012
- - - -
INTRODUCTION
Groundwater
is a precious and the most widely distributed resource of the earth and unlike
any other mineral resource, it gets annual replenishment from the meteoric
precipitation. The worlds total water resources are estimated at 1.37 x 108
Million hectares (ha-m). Of these global water resources about 97.2% is
salt water mainly in oceans, and only 2.8% is available as Fresh water. Out of
the 2.8%, 2.2% is available as surface water and 0.6% as groundwater. Even out
of this 2.2% of surface water, 2.15% is fresh water in glaciers and ice caps
and only of the order of 0.01% (1.36x104 M ha-m) is available in
lakes and reservoirs and 0.0001% in streams. Out of 0.6% of stored groundwater,
only about 0.3% (41.1 x 104 M ha-m) can be economically extracted
with the present drilling technology, the remaining is unavailable as it is
below 800 m.
Thus
groundwater is the largest source of fresh water on the planet excluding the
glaciers and the ice caps. The amount of groundwater within 800m from the
ground surface is 30 times the amount of all fresh water lakes and reservoirs
and about 3000 times the amount in stream channel at any one time.
At
present nearly one fifth of all the water used in the world is obtained from
groundwater resources. Agriculture is the greatest user of water accounting for
80% of all consumptions. It takes, roughly speaking, 1000 tons of water to grow
1 ton of grain, and 2000 tons to grow one ton of rice. Animal husbandry and
flasheries all require abundant water. Some 15% of world’s crop land is
irrigated. The present irrigated area in India is 60 million hectares (M ha).
Of this 40% is from ground water (H.M. Ragunhath, 2007).
Knowledge
on hydrochemistry of groundwater is essential for understanding its suitability
and optimum usage for domestic, industrial and agricultural purposes.
AIM
AND OBJECTIVE
This
project was undertaken by (three) Environmental Science Students of Yuvaraja’s College,
University of Mysore, to evaluate and asses the quality of groundwater for
drinking purpose of Sindhuvalli Village, Mysore District.
STUDY
AREA
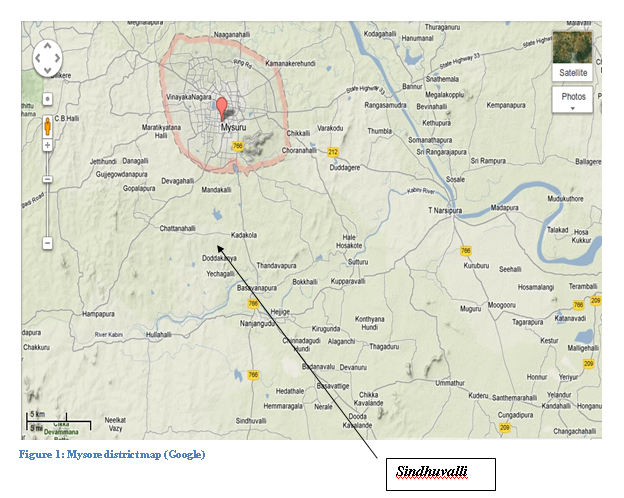
.JPG) The
Study area is located in Sindhuvalli, it is one of the villages in Mysore
Taluk , Mysore District , Karnataka State. Sindhuvalli is 13 km distance from
its District Main City Mysore and 135 km distance from its State Main City
Bangalore. It’s Neighbouring villages are Kadakola (3.1 k.m.), Udbur
(4.8 k.m.), Thandavapura (5.4 k.m.), Maraluru (6.5 k.m.), Srirampura
(6.5 k.m.). Nearby towns are Nanjangud (9.7 k.m.), Tirumakudal-Narsipur
(29.8 k.m.), Heggadadevankote (35.5 k.m.), Krishnarajanagara (38.5
k.m.).
The
Study area is located in Sindhuvalli, it is one of the villages in Mysore
Taluk , Mysore District , Karnataka State. Sindhuvalli is 13 km distance from
its District Main City Mysore and 135 km distance from its State Main City
Bangalore. It’s Neighbouring villages are Kadakola (3.1 k.m.), Udbur
(4.8 k.m.), Thandavapura (5.4 k.m.), Maraluru (6.5 k.m.), Srirampura
(6.5 k.m.). Nearby towns are Nanjangud (9.7 k.m.), Tirumakudal-Narsipur
(29.8 k.m.), Heggadadevankote (35.5 k.m.), Krishnarajanagara (38.5
k.m.).
The
village has a population of over 2000 people. One of the main sources of water
for villagers is through bore wells. Villagers opt to drink straight from the
bore-wells; no water treatment is carried out except boiling for drinking
purpose.
Study
area maps:
 |
| Figure 2: Bore well locations in the village |
MATERIALS
AND METHODS
For the present study,
during April 2012, 5 groundwater samples were collected from bore wells into pre-cleaned plastic sampling bottles (1 litre
capacity), after pumping out water for about 10 minutes to remove stagnant
water from the bore wells.
Electrical Conductance
and pH were measured in the field using digital pH meter.
Chemical analysis of
the water samples were carried out at Yuvaraja’s College, Environmental Science
Laboratory. During the chemical analysis, Calcium (Ca2+ ) and Magnesium
(Mg2+ ) were analyzed titrimetrically using standard Ethylenediaminetetraacetic
acid (EDTA). Na+ and K+ were
determined by flame photometry. HCO3-
and Cl- were estimated using acid titration method. SO42- was determined
using Nephlometer. NO3-
was determined by titrimetric method using FAS (Ferric Ammonium Sulphate). Total
Hardness (T H) was also calculated, TDS, EC and pH parameters were measured
using the digital pH meter.
RESULTS
AND DISCUSSION
Table of results:
Table 1. Physico-chemical
characteristics of the groundwater
Sample No.
|
pH
|
EC
|
TH
|
TDS
|
Ca2-
|
Mg2+
|
Na+
|
K+
|
HCO3-
|
SO42-
|
Cl-
|
NO3-
|
1
|
6.86
|
1262
|
420
|
898
|
265.67
|
145.66
|
77
|
80
|
360
|
23
|
120
|
671.6
|
2
|
7.27
|
644
|
200
|
457
|
60.00
|
67.63
|
27
|
Nil
|
480
|
29
|
140
|
64.4
|
3
|
7.07
|
713
|
200
|
510
|
85.73
|
52.02
|
40
|
Nil
|
400
|
20
|
280
|
524.4
|
4
|
6.72
|
2.36
|
490
|
1.67
|
222.89
|
119.65
|
145
|
70
|
340
|
34
|
300
|
432.4
|
5
|
6.73
|
2.40
|
580
|
1.68
|
188.60
|
187.28
|
146
|
185
|
350
|
30
|
257
|
763.6
|
Table 2. Assessment of
the suitability of the groundwater of the study area for drinking purposes
Range
|
WH0(2011 &1993)
|
US-EPA (2002)
|
BIS
(1991)
|
||
pH
|
6.72 – 7.27
|
6.5 - 8.5
|
6.5 - 9.2
|
6.5 -8.5
|
|
EC(μs/cm)
|
644µs – 2.40ms
|
1400
|
-
|
-
|
|
TH(mg/L)
|
200 – 580
|
500
|
-
|
-
|
|
TDS(mg/L)
|
1.68 – 898
|
1000
|
1500
|
2000
|
|
Ca2+(mg/L)
|
60 - 265.67
|
200
|
200
|
200
|
|
Mg2+(mg/L)
|
52.02 – 187.28
|
50
|
150
|
100
|
|
Na+(mg/L)
|
27 – 146
|
200
|
200
|
-
|
|
K+(mg/L)
|
70 – 185
|
12
|
25
|
-
|
|
HCO3-(mg/L)
|
340 – 480
|
-
|
600
|
600
|
|
SO42-(mg/L)
|
20 – 34
|
250
|
400
|
400
|
|
Cl-(mg/L)
|
120 – 300
|
250
|
250
|
1000
|
|
NO3-(mg/L)
|
64.4 – 763.6
|
50
|
50
|
-
|
|
WHO:
Word health organization; US-EPA: United States environmental protection agency; BIS: Bureau of Indian standards;. * If so42- is <=
250, then mg2+ >30 <100 mg/l will be considered as permissible limit.
Results and discussion:
In
the total five samples, two samples are exceeding with calcium values when
compared with the WHO (World Health Organization) standards. Calcium occurs
most commonly in sedimentary rocks in the minerals calcite, dolomite and
gypsum. It also occurs in igneous and metamorphic rocks chiefly in the silicate
minerals.
With
respect to Magnesium, all the samples are exceeding WHO standards. This shows
the water is hard and not much suitable for drinking without water treatment.
A
large number of minerals contain magnesium, for example dolomite. Magnesium is
washed from rocks and subsequently ends up in water. Magnesium has many
different purposes and consequently may end up in water in many different ways.
Chemical industries add magnesium to plastics and other materials as a fire
protection measure or as filler. It also ends up in the environment from
fertilizer application and from cattle feed. Human body contains about 25 g of
magnesium, of which 60% is present in the bones and 40% is present in muscles
and other tissue. Magnesium and calcium often perform the same functions within
the human body and are generally antagonistic. There are no known cases of
magnesium poisoning. At large oral doses magnesium may cause vomiting and
diarrhea.
In
all samples, Potassium exceeds WHO standard, this may be due to infiltration of
Potash used in fertilizers. Adverse health effects due to potassium consumption
from drinking-water are unlikely to occur in healthy individuals. Potassium
intoxication by ingestion is rare, because potassium is rapidly excreted in the
absence of pre-existing kidney damage and because large single doses usually
induce vomiting (Gosselin, Smith & Hodge,1984).
Three
samples are exceeding in Chloride content. This enrichment of chloride is
attributed by both natural and anthropogenic sources, such as run-off
containing road de-icing salts, the use of inorganic fertilizers, landfill leachates,
septic tank effluents, animal feeds, industrial effluents and irrigation
drainage. Chloride increases the electrical conductivity of water and thus
increases its corrosivity.
Chloride
toxicity has not been observed in humans except in the special case of impaired
sodium chloride metabolism, e.g. in congestive heart failure.
All
five samples are exceeding in Nitrite content. Nitrate can reach both surface
water and groundwater as a consequence of agricultural activity (including
excess application of inorganic nitrogenous fertilizers and manures), from
wastewater treatment and from oxidation of nitrogenous waste products in human
and animal excreta, including septic tanks. Höring & Schiller (1987), Sauerbrey
& Andree (1988), and van Maanen et al. (1994) found that inorganic nitrate
in drinking-water is a manifested factor of endemic goiter (swelling in the
thyroid gland).
.JPG) |
| Figure 4: Children use bore wells |
CONCLUSION
By comparing all the values of the physico-chemical parameters with WHO standards, it is evident that these bore well samples are not suitable for drinking without treatment of water. We recommend authorities to insure that safe drinking water is provided to the inhabitants of the village. It is essential that water be purified (Reverse osmosis technology) before used for drinking purpose etc. Further assessments need to be carried out in the area with more bore-well samples and study.
References:
H.M Ragunanth (2007), Ground Water , Third Edition, New Age
International (P) Ltd.
Dr. Rajashekhara Shetty
(2009), An Analysis of World Resources
with reference to India, SARALA RAJ, RIA publishers.
WHO (2007), Nitrate and nitrite in drinking-water, Background
document for development of
WHO
Guidelines for Drinking-water Quality, World Health
Organization (Research paper)
WHO (2009), Potassium in Drinking-water, Background
document for development of WHO Guidelines for Drinking-water Quality, World
Health Organization (Research paper)
WHO (2003), Chloride in Drinking-water, Background
document for development WHO Guidelines for Drinking-water Quality, World
Health Organization (Research paper)
Yuvraja's College, University of Mysore Environment Science Research and Lab procedures.
- - - - -